
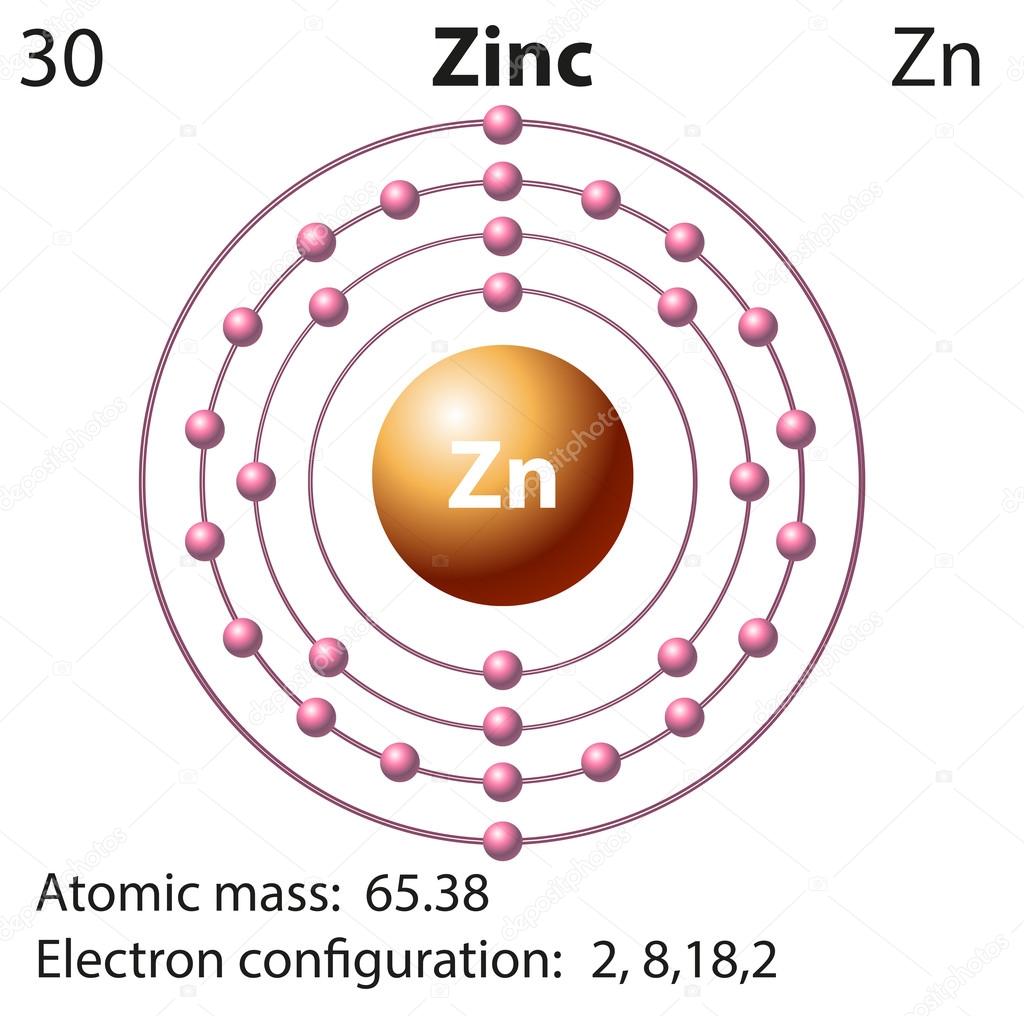
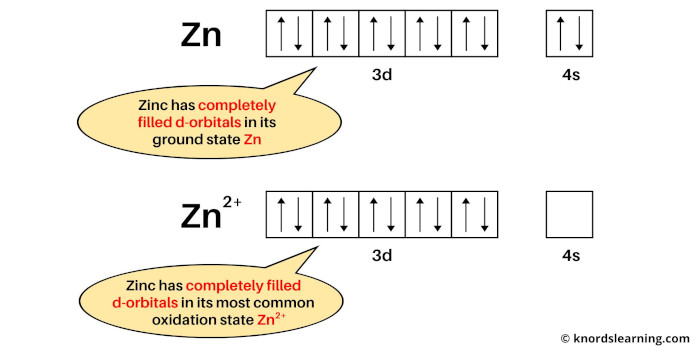
The N shell containing 4s, 4d, 4p and 4f, can carry 32 electrons. The M shell contains 3s, 3p, and 3d, and can carry 18 electrons. The K shell contains a 1s subshell hence it can carry 2 electrons, the L shell has 2s and 2p, and can carry 8 electrons. This decides the electron capacity of the shells. The maximum electrons that can be carried by the sub-shell S is 2, by P is 6, by D is 10, and the F sub-shell can carry 14. Zinc X-ray photoelectron spectra, zinc electron configuration, and other elemental information. Each shell and subshell have a limitation on the amount of electrons that it can carry. The subshells have a distinct shape and configuration, in which the electrons move freely. We have to remove two electrons from zinc. Zn electronic configuration 1s 2 2s 2 2p 6 3s 2 3p 6 4s 2 3d 10. The given ion Zn 2+ 2+ charge indicates substraction of two electrons from neutral electronic configuration. They stand for sharp (S), principal (P), diffuse (D), and fundamental (F). If an ion has a negative charge, we have to add the electrons from the neutral electronic configuration of the element. The shells are labeled K, L, M, N, and so on, from the innermost to the outermost shell.Įach shell has subshells that are named for the type of emission lines produced from different states of angular momentum. This model has been widely accepted, and according to it, each atom has shells, which further have subshells. It involves the specific arrangement of electrons in shells and sub-shells of Bohr’s atomic model. The concept of electronic configuration has replaced the older concept of valency and valence electrons. The electronic configuration of each element is decided by the Aufbau principle which states that the electrons fill orbitals in order of increasing energy levels. ZINC Electronic configuration: Ar 3d10 4s Formal oxidation number: +2 Electronegativities: 1.65 Atomic radius / pm: 133.5 Relative atomic mass: 65.38(2).


 0 kommentar(er)
0 kommentar(er)
